ZilrettaTM for Osteoarthritis
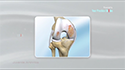
Zilretta (triamcinolone acetonide extended-release injectable suspension) is a US Food and Drug Administration-approved treatment to manage osteoarthritis pain within the knee joint. It is available in 32mg (5ml)-dose vials.
Zilretta is the first and only FDA-approved treatment for osteoarthritic knee pain to employ extended-release microsphere technology. Microspheres are tiny particles invisible to the eye. The microspheres in Zilretta comprise a medicine called triamcinolone acetonide that assists in reducing the inflammation and pain caused by osteoarthritis. Once injected directly into the knee, the microspheres remain at the site, continually and slowly releasing medicine for about 3 months.
Disease Overview
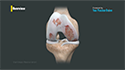
Osteoarthritis of the knee is a degenerative joint disease that may lead to intermittent discomfort and ultimately to severe chronic pain, loss of function, and irreversible structural damage. The risk of osteoarthritis normally increases after age 45; however, it can also occur in younger individuals. The symptoms include stiffness, pain when moving the knee, crepitus (a crunching, creaking, or grinding sensation with movement), and swelling due to additional fluid in the joint.
Indications
Zilretta is indicated for the treatment of osteoarthritic knee pain that has not responded to traditional conservative treatments such as anti-inflammatory medications, physical therapy, activity modification, ice therapy, or immediate-release corticosteroid or hyaluronic acid injections.
Preparation
The pre-procedure preparation for the Zilretta intra-articular injection may involve the following:
- A review of your medical history and a physical examination
- Routine blood work and imaging
- Informing your doctor about medications or supplements you are taking
- Informing your doctor about allergies to any medications, anesthesia, or latex
- Disclosing any recent illnesses or other medical conditions
- Refraining from medications such as blood-thinners and nonsteroidal anti-inflammatories, or other supplements, if contraindicated for the procedure
- Arranging for someone to drive you home after the procedure
Procedure
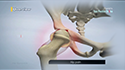
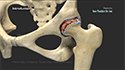
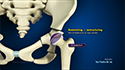
During the Zilretta intra-articular knee injection procedure, you will be seated or will lie on your back with the affected knee flexed or extended. The skin over the injection site is sterilized and numbed with a local anesthetic. A needle is inserted into your knee joint and the Zilretta medication is injected. In some cases, ultrasound imaging is used to help guide the needle to the correct site. You may feel some mild discomfort during the injection. In some cases, prior to injecting the medicine, a small amount of joint fluid is withdrawn to make space for the medication. Once the fluid is removed, the same site is used to administer the intra-articular injection. A small dressing is then applied over the injection site to complete the procedure.
Post-procedure care
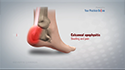
Following the procedure, you will be kept under observation for a few hours before being discharged. You should be able to fully weight-bear after a couple of hours of resting. Crutches may be needed in the short term to enable the joint to settle down and to facilitate walking. You may notice pain, swelling, or discomfort in the treatment area. Over-the-counter medications are provided to address these symptoms. You may also apply ice to the injection site for additional comfort. Avoid strenuous activities for a couple of days. Gentle range-of-motion exercises are recommended to optimize knee function.
Risks and Complications
Some of the adverse effects associated with a Zilretta injection include:
- Allergic reaction
- Sore throat
- Runny nose
- Bruising
- Coughing
- Headache
- Back pain
- Pain and swelling in the joint
Summary
Zilretta is a safe and well tolerated intra-articular injection for osteoarthritis pain of the knee. It contains triamcinolone acetonide, a synthetic corticosteroid with anti-inflammatory and immunomodulating properties, that has demonstrated significant reduction in pain intensity scores from weeks 1 through 12, making it an effective choice for osteoarthritic knee pain.